-
Secure Checkout
-
International Delivery
-
Quality-Checked
-
24X7 Customer Support
Manufacturer: Array Biopharma
| Brand Name | |
|---|---|
| Country Of Origin |
United States |
| Medicine Form |
Capsule |
| Medicine Type |
Generic |
| Molecule | |
| Pack Quantity |
42 Capsules |
| Packaging Type |
Bottle |
| Rx Status |
Rx (Prescription Medicine) |
| Strength |
75 mg |
Introduction to Braftovi Encorafenib Braftovi (Encorafenib) is a targeted therapy. It is designed to treat cancers with the BRAF V600 mutation. This genetic alteration can cause uncontrolled cell growth and tumor formation. Braftovi is manufactured by Pierre Fabre. It is a kinase inhibitor. It works by blocking the abnormal BRAF protein. This protein plays a key role in cancer cell growth. Braftovi is used in combination with other treatments. It is used for conditions like melanoma and colorectal cancer. Braftovi is available in 75 mg capsules. It is prescribed for advanced melanoma and metastatic colorectal cancer. These cancers must have the BRAF V600E mutation. Braftovi targets a key mechanism in cancer cell signaling. It helps slow tumor growth and spread. This offers hope for patients with difficult-to-treat cancers. How Does Braftovi Work? Braftovis active ingredient is Encorafenib. It is a highly potent BRAF inhibitor. Encorafenib selectively targets and inhibits the BRAF V600E and V600K mutations. In cancers with these mutations, the BRAF protein becomes defective. This leads to the activation of the MEK/ERK signaling pathway. The MEK/ERK pathway promotes excessive cell division and growth. This pathway is also known as the MAPK pathway. Its activation is a common cause of tumorigenesis in various cancers. By blocking BRAF activity, Braftovi prevents downstream signaling in the MAPK pathway. This halts cancer cell proliferation. It also reduces the tumors ability to grow and spread. Braftovi is used with other therapies. For melanoma, it is used with Mektovi (binimetinib). For colorectal cancer, it is used with Cetuximab. Braftovi enhances the effectiveness of treatment. It targets multiple points in the cancer cell signaling pathway. This combination therapy approach tackles the tumor from different angles. It improves the overall therapeutic outcome. It reduces the likelihood of resistance to treatment. The result is more effective control of tumor progression. This gives patients a better quality of life. In some cases, it may prolong survival. Uses of Encorafenib Braftovi (Encorafenib) is approved for the treatment of patients with cancers that exhibit the BRAF V600E or V600K mutations. It is commonly prescribed for: 1. Melanoma (Unresectable or Metastatic) Braftovi is used in combination with Mektovi (binimetinib) to treat unresectable or metastatic melanoma with a BRAF V600E or V600K mutation. Melanoma is a form of skin cancer that can be aggressive and spread to other parts of the body. Braftovi is designed to treat this type of cancer when it cannot be surgically removed or when it has spread to other areas (metastasized). 2. Colorectal Cancer (CRC) (Metastatic) Braftovi is also indicated in combination with Cetuximab for the treatment of metastatic colorectal cancer in patients who have the BRAF V600E mutation. Colorectal cancer is a common cancer that can spread to distant organs, making it difficult to treat. Braftovi helps by inhibiting the growth of cancer cells with the BRAF mutation, providing a targeted therapy option for patients who have previously undergone treatment. Braftovi has been approved by the FDA , EMA, and PMDA in various countries, making it a critical option for patients with BRAF-mutant cancers. The drug has been granted special designations such as Priority Review and Breakthrough Therapy by regulatory agencies due to its promising therapeutic outcomes. It is important to consult your doctor regularly for any necessary adjustments to your treatment regimen, particularly if you are experiencing side effects or taking medications that may interact with Braftovi. Benefits of Braftovi Braftovi is a precision medicine. It specifically targets the BRAF mutation. This helps direct treatment at the root cause of cancer cell growth. When used with Mektovi (binimetinib) for melanoma or Cetuximab for colorectal cancer, Braftovi works synergistically. It blocks the cancer’s ability to grow and spread. This provides a more robust and effective treatment option. In clinical trials, Braftovi, combined with other drugs, showed significant improvements in progression-free survival (PFS). This was seen in patients with BRAF-mutated melanoma and colorectal cancer. By targeting multiple pathways in cancer cell signaling, Braftovi reduces the chances of cancer cells developing resistance. Resistance is a common issue in cancer therapies. Braftovi comes in capsule form. It is easy for patients to incorporate into their daily routine. It is typically taken once a day, with or without food. Patients take it for as long as the disease does not progress, or side effects remain manageable. Side Effects Like all medications, Braftovi may cause side effects, though not everyone will experience them. The severity and type of side effects can vary from patient to patient. Below are the most commonly reported side effects of Braftovi when used in combination with Mektovi (binimetinib) for melanoma and Cetuximab for colorectal cancer: Common Side Effects Fatigue Nausea and Vomiting Abdominal Pain Arthralgia Diarrhea Dermatitis Acneiform Decreased Appetite Serious Side Effects Skin Cancers Heart Problems Liver Problems Eye Problems Warnings and Precautions Pregnancy and Breastfeeding Braftovi can be harmful to a fetus and should not be used during pregnancy. Women who are pregnant or breastfeeding should avoid using Braftovi. Effective contraception should be used during treatment and for at least two weeks after the final dose. Drug Interactions Braftovi may interact with medications that affect the CYP3A4 enzyme, which plays a role in metabolizing drugs. It is important to inform your doctor about any other medications, including over-the-counter drugs and supplements, that you are taking.
Only logged in customers who have purchased this product may leave a review.
Understand what medicines you can legally import in your Country
Medicine import rules vary by country and change frequently.
Before ordering or shipping medicines internationally, it’s important to understand the legal, documentation, quantity, and customs requirements applicable to your destination. We help you stay informed by sharing up-to-date, country-specific medicine import guidelines directly to your email and WhatsApp — so you can avoid delays, rejections, or compliance issues.
We work with reliable, internationally recognised partners to ensure safe and timely delivery of your order.
Loading…
*Delivery timelines are indicative and may vary depending on destination country, customs clearance, public holidays, or unforeseen logistics delays.
Processing time: 1–2 working days
Once your order and payment are confirmed, our team verifies the prescription (if required), prepares the package, and hands it over to the respective logistics partner within 1–2 working days.
Orders placed on weekends or public holidays will be processed on the next working day.
For transparency and peace of mind, IndogenMed provides parcel photos showing how your medicines are packed before dispatch.
Outer parcel packaging

Inner packaging layer

Secure labeling and packing

Sealed package ready for dispatch
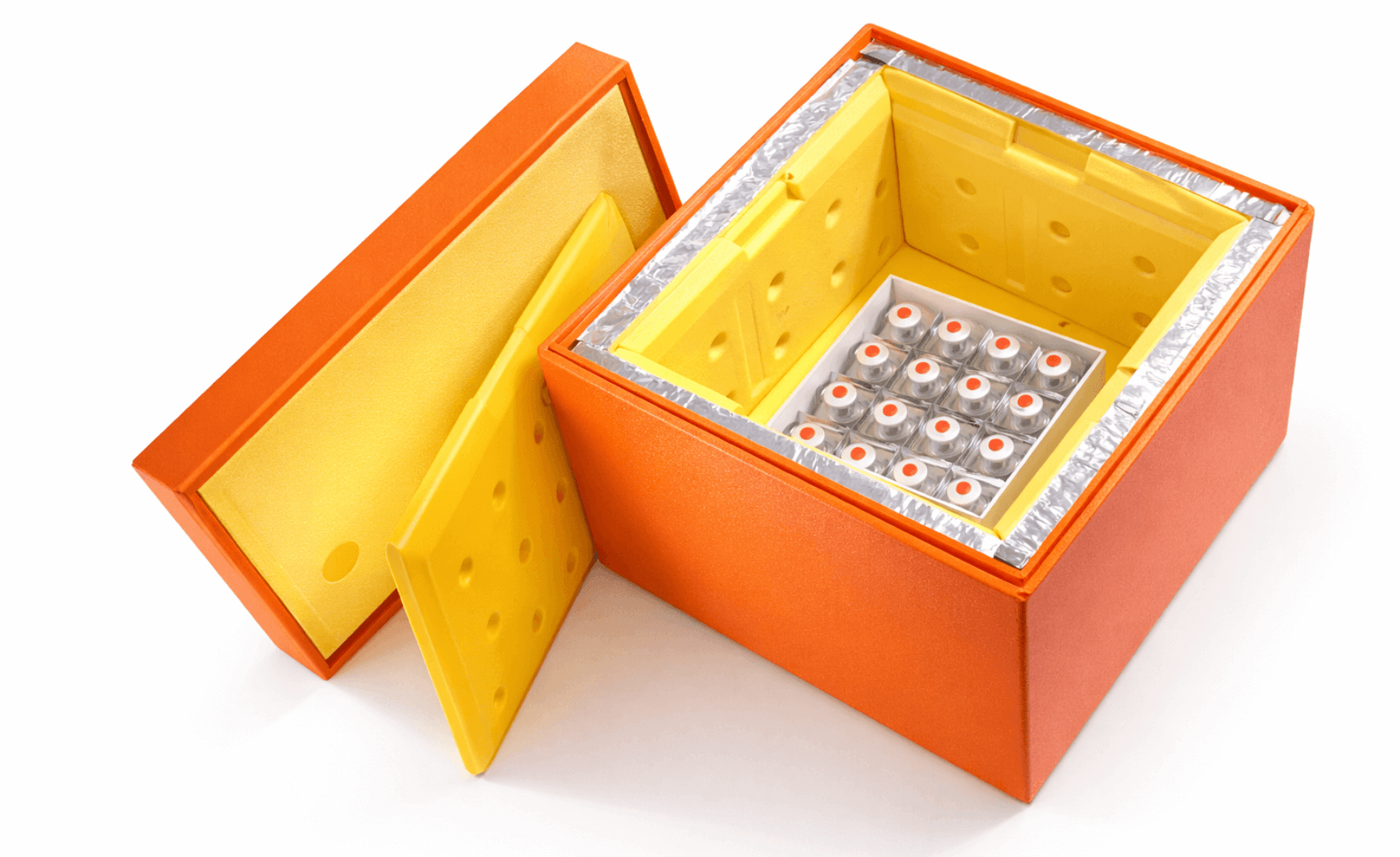
Cold chain insulated box

Final courier handover
Actual parcel appearance may vary based on order size, destination, and courier requirements.
You can share your prescription via:
Your shared details and health information are completely safe and secure. Even IndogenMed employees cannot access your documents freely.
We comply with Indian IT data protection laws and global data privacy standards.
Submit your details below and we’ll send you official medicine import guidelines for your selected country
Submit your details and we’ll get back to you with the right information.

wyatt whitney –
Review from OpenCart migration